Cis trans isomerism
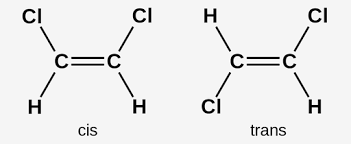
Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively.
This occurs in compounds with double bonds.
Since the double bond is rigid , the atoms attached to it are not free to rotate as are those attached to a single bond.
Maleic acid cis
Fumaric acis trans
These 2 structures are not equivalent and have different chemical and physiological properties.
Fumaric acid but not maleic acid is physiologically active.
The cis isomer / maleic acid has 2 more bulky groups ( COOH) on the same side of the double bond.
If they are on the opposite side of the double bond , a trans isomer is produced.
Introduction of trans double bonds in an otherwise saturated hydrocarbon chain deforms the shape of the molecule relatively little.
A cis double bond by contrast entirely changes its shape.
It can this be appreciated why cis and trans isomers of a compound are not interchangeable in cells.
Membranes composed of trans and cis isomers would have entirely different shapes.
Enzymes acting on one isomer would be expected to be entirely inert with the other.
The usual formulas fail to represent the actual shape of the molecules.
Portions of the hydrocarbon backbone of a saturated fatty acid and of the cis and trans isomers of an 18 carbon unsaturated fatty acid are represented in Fig 1-7
Cis-trans isomers exhibit a type of stereoisomerism where the atoms have different spatial arrangements in three-dimensional space. In the field of organic chemistry, cis isomers contain functional groups on the same side of the carbon chain whereas the functional groups are on opposite sides in trans isomers.
Why trans isomers are more stable?
Trans isomer is more stable than cis isomer because in cis isomer, the bulky groups are on the same side of the double bond. The steric repulsion of the groups makes the cis isomer less stable than the trans isomer in which the bulky groups are far apart ( They are on the opposite side of the double bond).

No comments:
Post a Comment